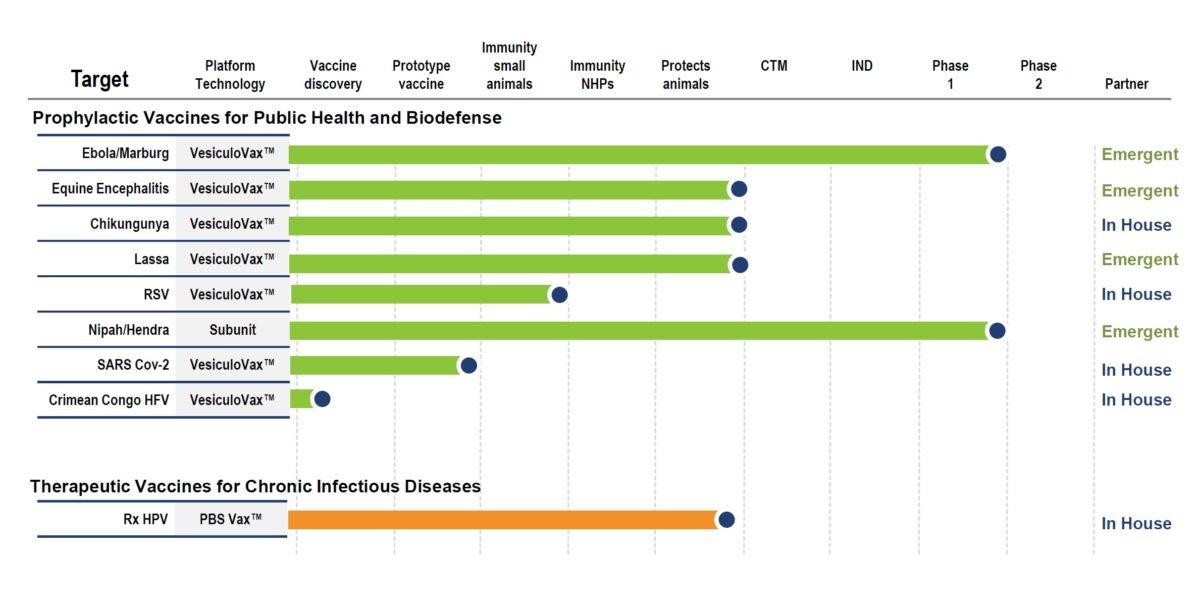
The Auro Vaccines discovery engine is rapidly creating a pipeline of differentiated new vaccines. On the leading edge of development are:
- A clinical-stage vaccine franchise for the Ebola and Marburg viruses that has received substantial funding support from the federal government which has been out licensed to Emergent BioSolutions for further clinical development
- A Nipah/Hendra virus subunit vaccine currently in Phase I clinical evaluation in collaboration with the Coalition for Epidemic Preparedness Innovation and PATH
- Prophylactic vaccines for the prevention SARS CoV-2 and Chikungunya virus infection which are poised to enter clinical evaluation
- A therapeutic vaccine for the treatment of cervical cancer caused by Human papilloma virus infection which is poised to enter clinical evaluation